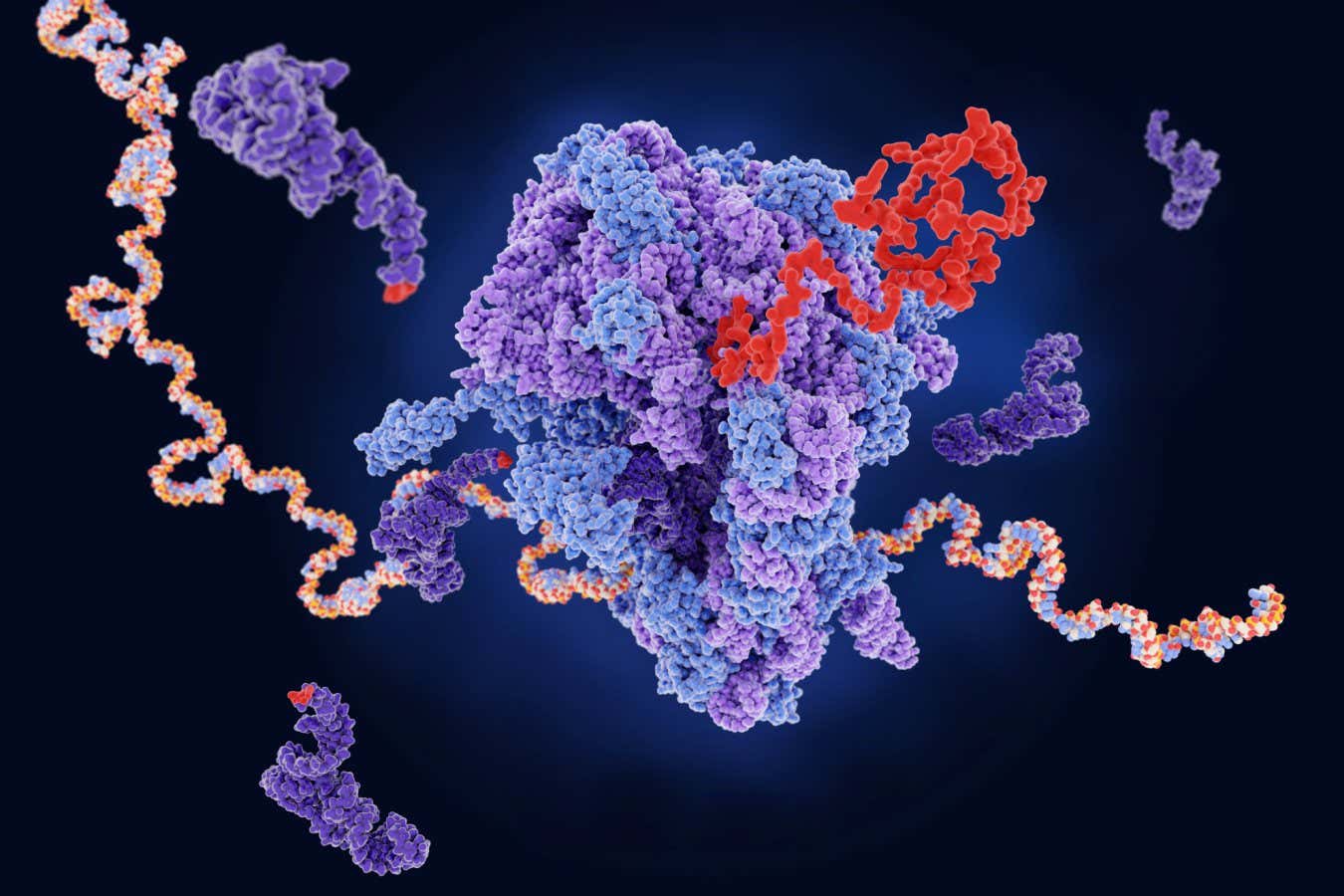
A ribosome (centre) producing a protein (red) from mRNA. The dark purple strands represent transfer RNA, which are also involved in protein production
We may have discovered a fundamental cause of cellular ageing that underlies many other ageing processes in cells.
A study of the brains of freshwater fish called killifish has shown that, as they age, the protein-making factories in cells start jamming while making a key class of proteins, causing a vicious cycle of decline.
The discovery could lead to new ways to tackle brain ageing, says Alessandro Cellerino at the Leibniz Institute on Aging in Germany. “We are mostly talking about improving cognition or preventing cognitive decay, rather than increasing lifespan,” he says.
The recipes for making proteins are stored in DNA in our cells. When a protein is needed, copies of these recipes are transcribed into a kind of molecule called mRNA.
The mRNA copies are edited, or spliced, and sent to protein-making factories called ribosomes, which bind to the mRNA molecules and move along them, reading off the three-letter codons and translating them into a sequence of amino acids that make up the protein.
Normally, the more mRNA copies there are, the more of a protein is produced. But a growing number of studies have shown that, as human cells age, this correlation breaks down, so the production of a protein can decline despite no reduction in the amount of mRNA.
Cellerino and his team may now have found out why this happens by studying ribosomes in the brains of killifish as they age. The researchers used a technique that allowed them to take a snapshot of how far each ribosome had moved along the mRNA it was bound to.
What they found is, as the killifish’s brains aged, there were a lot more ribosomes bound to the codons specifying the amino acids arginine and lysine than would be expected by chance. This means the ribosomes are stalling at these codons, halting production before the protein is complete.
Arginine and lysine are both positively charged amino acids that are abundant in proteins that bind to DNA or RNA, which are both negatively charged. This means it is these DNA- and RNA-binding proteins that are most likely to be affected by the stalling.
That is a problem, because these proteins carry out key functions, such as making RNAs, splicing RNAs, repairing DNA damage and so on.
“It is known that with ageing, there is DNA damage, there is less production of RNA, there is less splicing, there is less production of proteins,” says Cellerino. “What we suggest is that this phenomenon of ribosome stalling connects all these different hallmarks of ageing.”
What’s more, ribosomes themselves contain RNA-binding proteins, he says. “So there is this vicious cycle by which there is stalling on the mRNAs that code for ribosomal proteins, which results in less production of ribosomes, which results in less protein synthesis.”
The big question now is whether ribosomal stalling happens in human brains. Earlier this year, Gene Yeo at the University of California San Diego showed that RNA-binding proteins become depleted in human neurons as they age. To this extent, his findings are in agreement with Cellerino’s, he says, but the cause isn’t yet clear. “We are working out why RNA-binding proteins are altered.”
If the findings do apply to people, it could lead to new treatments for age-related brain conditions. In killifish, the stalling of ribosomes also triggers an alarm signal that produces an inflammatory response. “The constant activation of this pathway causes chronic inflammation,” says Cellerino. “Chronic inflammation is a very important factor in ageing, especially in the brain.”
There are experimental drugs that can block this signalling pathway and thus might help stave off such conditions, says Cellerino.
“But for lifespan, it is too early to really say anything,” he says. This is because the reason why ribosomes start stalling on certain amino acids isn’t yet understood, and it also isn’t clear whether the same stalling process happens in all organs.
Topics: