Ever wonder what happens to those old Teflon-coated frying pans? Not a lot. Most get chucked into landfills. However, a new recycling technique developed by Japan’s National Institutes for Quantum Science and Technology (QST) promises 100% effectiveness.
Teflon is one of science’s wonderful examples of serendipity. In April 1938, DuPont chemist Roy J. Plunkett was working on a new, safer chlorofluorocarbon refrigerant that wasn’t explosive or poisonous like the ones used in then-current refrigerators.
He was mucking about with tetrafluoroethylene (TFE) gas mixed with hydrochloric acid to see how they’d react when he discovered that one cylinder that had been stored on dry ice seemed to be empty, though it still weighed as much as if it was full. Cutting it open, he found a slippery white powder that turned out to be the TFE spontaneously polymerized, turning it into polytetrafluoroethylene (PTFE).
What we now know under its trade name, Teflon.
At first, the expensive-to-produce chemical was strange, but no one could figure out what to do with it. It was fantastically inert from a chemical point of view. Nothing could dissolve or degrade it. It didn’t interact with living tissue. Water and oil, in fact almost nothing, would stick to it, with liquids beading up and running off. It was also nearly frictionless, maintained its chemical and physical properties over a wide range of temperatures, was an excellent electrical insulator, wouldn’t burn, and it was durable and flexible.
It was the classic case of a solution in search of a problem.
Teflon probably would have ended up on a DuPont lab shelf and remained an obscure entry in the Handbook of Chemistry and Physics if it hadn’t been for the Manhattan Project to build the first atomic bomb. Long story short, the scientists at Oak Ridge tasked with refining the uranium needed for the bomb were having problems with the highly corrosive uranium hexafluoride used to separate the uranium isotopes.
For reasons that are still largely classified, what was still called polytetrafluoroethylene was just the ticket for protecting valves, pipes, and other equipment while keeping them free from residue. So, the strange powder got a chemical foot in the door.
By the 1950s, Teflon had its official name and was finding all sorts of applications in chemical works, electronics, engineering, and other applications. It ended up being used in food processing, wire insulation, circuit boards, bearings, bushes, seals, valves, catheters, capacitors, buildings, textiles, and aircraft components.
Then, in the early 1960s, it burst onto the consumer market with the first non-stick frying pans, which became extraordinarily popular despite their tendency to scratch if you looked at them wrong and the aluminum in the pans being rubbish for heat retention, but that’s just the opinion of someone who much prefers seasoned cast iron. A lot.
This chemical success story is all well and good and we could end it there if it weren’t for one nagging problem. The very properties that make Teflon a miracle plastic also make it incredibly difficult to recycle. In fact, most recycling programs won’t take Teflon pans and they end up in landfills.
It is possible to recycle Teflon, but it’s a long, difficult process that can involve separating the Teflon from whatever it’s coating, grinding it up, subjecting it to high temperatures, and then chemicals. The heating alone makes it a very energy intensive and expensive job.
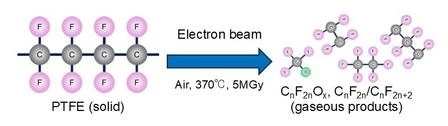
Now, the QST team has come up with a new method to tackle Teflon based on using an electron beam to break down the plastic’s molecules. This is done by heating the Teflon in air to a temperature of 370 °C (698 °F), then zapping it with a 5-MGy electron beam. This breaks down the polymer with 100% efficiency by altering the internal structure of the molecules to produce oxidized fluorocarbons and perfluorocarbons that can be recycled to make new products.
QST
It also means significant cost savings and much lower atmospheric emissions because the electron beam uses only half the energy of the conventional high-temperature pyrolysis method.
The hope now is to take the new recycling process out of the laboratory and refine it so it can be scaled up to industrial levels.
“We hope this technology will contribute to the safer, cleaner, and more cost-effective recycling of high-performance plastics,” said Dr. Yasunari Maekawa, leader of the research project.
The research was published in Radiation Physics and Chemistry.
Source: QST
