Once dismissed as “junk,” a group of RNA molecules has been found to help regrow damaged nerves in mice in new research. The discovery could unlock new ways to treat nerve injuries and even restore growth in the brain and spinal cord.
Humans have limited regenerative abilities when it comes to repairing damaged nerves, especially in the central nervous system (the brain and spinal cord). Limited as it is, nerve regeneration in the peripheral nervous system is affected by age, injury severity and location, as well as the presence of certain molecules that can inhibit the process.
New research led by the Weizmann Institute of Science (WSI) in Israel has identified hundreds of RNA molecules that stimulated the growth of peripheral nerve cells and, when artificially introduced, also triggered regrowth in cells of the central nervous system of mice.
“There are still no effective treatments to accelerate nerve cell growth and regeneration,” said the study’s corresponding author, Professor Mike Fainzilber, from Weizmann’s Departments of Biomolecular Sciences and Molecular Neurobiology. While the growth acceleration observed in our study is not yet sufficient to address clinical paralysis, it is definitely significant.”

The researchers discovered that a special group of RNA molecules called growth-inducing Short Interspersed Nuclear Elements (GI-SINEs) play a key role in nerve growth following injury. These RNAs are a distinct subset of a larger family known as B2-SINEs, which are repetitive, non-coding elements in the genome. Repetitive, non-coding elements are stretches of DNA that repeat many times in the genome and don’t code for proteins themselves, but instead regulate the protein-making machinery inside cells. Once thought of as “junk” DNA and dismissed by scientists, the present study has shown that GI-SINEs actually activate a molecular program that helps nerve cells regrow after damage.
In essence, when a nerve is damaged, GI-SINEs are switched on in sensory neurons. They help increase protein production needed for the regeneration of the axon, the long, slender “cable” that projects from the neuron and transmits signals to other neurons, muscles, and glands. They do this in three ways:
- Being switched on by AP-1 transcription factors, which are proteins that control gene activity in response to stress or injury.
- Binding to ribosomes and nucleolin, which are crucial for protein synthesis.
- Helping reroute protein synthesis from the axon tips to the main cell body to boost regrowth.
The researchers employed a range of advanced laboratory techniques to investigate nerve regeneration. They used RNA sequencing (RNA-seq) to detect changes in RNA molecules following injury in sensory neurons (peripheral nervous system) compared to central neurons (central nervous system). Animal models of nerve injury, such as to the sciatic nerve (PNS) and optic nerve (CNS), were used to track GI-SINE expression and assess how these molecules influenced nerve regeneration. Viral gene delivery was employed to overexpress GI-SINEs and observe their effect on axon regrowth. Protein assays were conducted to identify the proteins that GI-SINEs bind to. Finally, antisense oligonucleotides (ASOs) were used to selectively block GI-SINEs and examine the resulting changes in neuron growth and protein interactions.
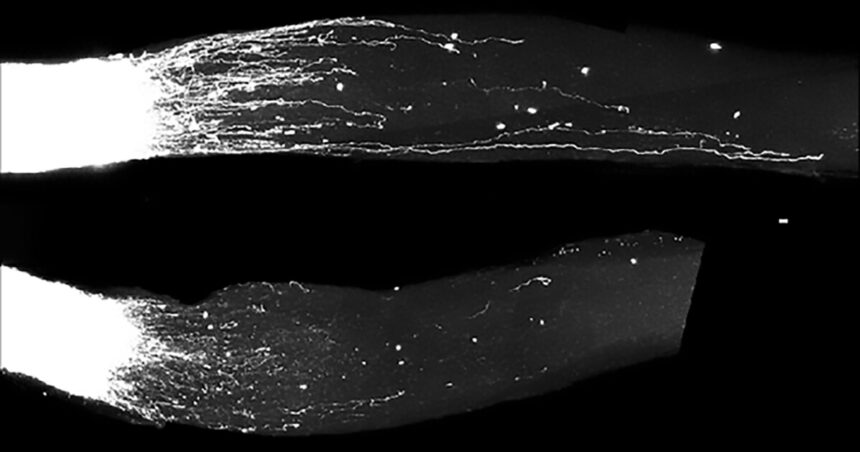
In mouse models, following sciatic nerve injury, the researchers found that GI-SINEs were turned on, which helped the neurons grow new axons. Natural regrowth occurred because the sciatic nerve is part of the peripheral nervous system, but it was boosted by GI-SINEs. When the researchers analyzed what happened after injury to parts of the central nervous system – retinal ganglion cells in the eye or the corticospinal tract in the spinal cord – they found that GI-SINEs weren’t naturally turned on. However, when the researchers artificially added GI-SINEs using gene therapy, these central neurons did start to regrow axons.

Zahavi et al./Weizmann Institute of Science
There are some limitations to the research. While GI-SINEs promoted axon growth in lab cultures and mouse models, the study doesn’t prove full nerve repair or recovery of function. It was shown that GI-SINEs interacted with ribosomes and nucleolin; they may also affect other processes not tested in this study. And, these elements were studied in mice. Similar elements may exist in humans, but this still need confirmation.
Nevertheless, the findings have huge potential, opening the door to new treatments for nerve injuries and neurodegenerative diseases. But there is still a long way to go.
“Of course, the path from basic research to clinical application is long, and we must make sure that enhancing growth mechanisms does not, for example, increase the risk of cancer,” Fainzilber said. “Recovery from peripheral nerve injuries, or from systemic diseases like diabetes that affect these nerves, can be very slow. That’s why we’re now testing a therapy that might speed up regeneration by mimicking B2-SINE activity.
“We are currently working with UCLA on a study showing that the mechanism we discovered plays a role in recovery from stroke in mouse models. Additionally, we’re collaborating with Tel Aviv University, Hebrew University and Sheba Medical Center to study its possible role in ALS, a progressive neurodegenerative disease. Neurodegenerative conditions affect many millions of people worldwide. While the road ahead is long, I truly hope one day we’ll be able to harness our newly discovered regeneration mechanism to treat them.”
The study was published in the journal Cell.
Source: Weizmann Institute of Science